OVERVIEW
Thalassemia are inherited blood disorders. It means that parents pass the genes for the disorder on to their children.
Thalassemia cause the body to make fewer healthy red blood cells and less hemoglobin than normal. Hemoglobin is an iron-rich protein in red blood cells. It carries oxygen to all parts of the body. Hemoglobin also carries carbon dioxide ( a waste gas) from the body to the lungs, where it’s exhaled.
People who have thalassemia can have mild or severe anemia. This condition is caused by a lower than normal number of red blood cells or not enough hemoglobin in the red blood cells.
Normal hemoglobin, also called hemoglobin A, has four protein chains (2 alpha globin and two beta globin). The two major types of thalassemia, alpha and beta, are named after defects in these protein chains.
Four genes (two from each parent) are needed to make enough alpha globin protein chains. Alpha thalassemia traits occur if one or two of the four genes are missing. If more than two genes are missing, moderate to severe anemia occurs.
Two genes (one from each parent) are needed to make enough beta globin protein chains. Beta thalassemia occurs if one or both genes are altered.
Two major types of thalassemia
Defect in the rate of synthesis of the alpha chains
Inheritance Pattern for α–Thalassemia .
Picture shows one example of how α thalassemia inherited.
The α globin genes are located on chromosome 16.
A child inherit 4 α globin – 2 from each parent.
Each child has 25% chance of inheriting either one of four possibility.
Alpha thalassemia occurs when one or more of the four genes needed for making the alpha globin chain of hemoglobin are missing. Moderate to severe anemia results when more than two genes are affected. The most severe form of alpha thalassemia is known as alpha thalassemia major. It can result in miscariage.
People with only one gene affected are called silent carriers and have no sign of illness. Deletion of one alpha gene, leaving three functional alpha genes. Borderline low of MCV (mean cell volume) 78 – 80 fL. No reliable way to diagnose silent carriers by hematologic methods. It must be done by genetic mapping. No hematologic abnormalities present.
q Two missing genes – Alpha thalassemia trait ( also called thalassemia minor or mild anemia)
People with two genes affected called as alpha thalassemia or alpha thlassemia minor. This people have mild anemia and are considered carriers. Two missing alpha genes maybe homozygous (-a/-a) or hetero zygous (--/aa). Sometimes it may be confused with iron deficiency anemia.
q Three missing genes – Hemoglobin H (moderate to severe anemia )
People with three genes affected have moderate to severe anemia or hemoglobin H disease. Usually caused by presence of only one gene producing alpha chains (--/-a). This unstable form causing the red blood cells to break down more quickly. Result is fewer red blood cell, a condition called anemia.
People with hemoglobin H disease do not have serious health problem but offer them to be more tired. However, these are rare except for children with hemoglobin H –Constant Spring disease which is a more severe form of this disorder.
Diagnosis: red blood cells are microcytic, hypochromic with marked poikilocytosis. Numerous target cells.
q Four missing genes – Bart’s Hydrops fetalis syndrome (alpha thalassemia major)
Babies with all four genes missing usually die before or shortly after birth. Most severe form and incompatible with life due to have no functioning alpha chain genes (--/--). Baby born with hydrops fetalis, which is edema and ascites caused by accumulation serous fluid in fetal tissues as result of severe anemia. Also see hepatosplenomegaly and cardiomegaly.
Hemoglobin Bart’s has high oxygen affinity so cannot carry oxygen to tissues. Fetus dies in uterus or shortly after birth. At birth, see severe hypochromic, microcytic anemia with numerous nucleated red blood cells. Pregnancies dangerous to mother. Increased risk of toxemia and severe postpartum hemorrhage.
....................................................................................................................................................................
Defect in the rate of synthesis of the beta chains
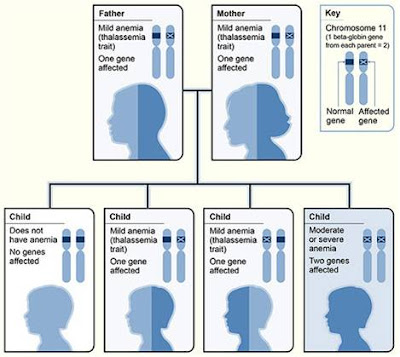
Inheritance Pattern for Beta Thalassemia
The picture shows one example of how β – Thalassemia is inherited.
The β globin gene is located on chromosome 11.
A child inherits two beta globin genes – one from each parents.
Two genes (one from each parent) are needed to make enough beta globin protein chains. If one or both of these genes are altered, you will have beta thalassemia. This means that you don’t make enough beta globin protein.
If you have one altered gene, you’re carrier. This condition is called beta thalassemia trait or beta thalassemia minor. It cuases mild anemia.
Various heterogeneous beta mutations that produce only small decrease in production of beta chains. Patients have nearly normal beta chain ratio and no hematologic abnormalities.
If one gene is affected (one normal beta gene and other mutated), a person is a carrier and has mild anemia. This condition is called beta thalassemia trait or beta thalassemia minor. Caused by heterogenous mutations that affect beta globin synthesis.
Diagnosis:
- Hemoglobin level in 10 – 13 g/dL range with normal or slightly elevated RBC count.
- Anemia usually with hypochromic and microcytic with slight anisocytosis and poikilocytosis.
- Rarely see hepatomegaly or splenomegaly.
- Normally required no treatment.
- Make sure are not diagnosed with iron deficiency anemia.
Cooley’s anemia or beta thalassemia major is a rare condition. Most of these persons had the severe forms of illness, but there may be more who are not diagnosed. Characterized by severe microcytic, hypochromic anemia.
Detected early in childhood:
- Infants fail to thrive
- Have pallor, variable degree of jaundice, abdominal enlargement and hepatosplenomegaly.
- Severe anemia causes marked bone changes due to expansion of marrow space for increased erythropoiesis.
- Characteristic changes in skull, long bones, and hand bones.
- Have protrusion upper teeth and Mongoloid facial features.
- Physical growth and development delayed.
- Peripheral blood shows markedly hypochromic, microcytic erythrocytes with extreme poikilocytosis.
.............................................................................................................
Laboratory Diagnosis
Complete Blood Count (CBC) with Differential
• Decrease in hemoglobin, hematocrit, mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH).
• Normal to slightly decreased mean corpuscular hemoglobin concentration (MCHC).
• Normal or elevated RBC count with a normal red cell volume distribution (RDW).
• Elevated RBC count with markedly decreased MCV differentiates thalassemia from iron deficiency anemia.
•On differential, see microcytic, hypochromic RBCs (except in carrier states). See mild to moderate poikilocytosis.
• In more severe cases, see marked number of target cells and elliptocytes. Will see polychromasia, basophilic stippling and nucleated RBCs.
Brilliant Cresyl Blue Stain
• Incubation with brilliant cresyl blue stain causes Hemoglobin H to precipitate.
• Results in characteristic appearance of multiple discrete inclusions – golf ball appearance of RBcs.
• Inclusions smaller than Heinz bodies and are evenly distributed throughout cell.
Acid Elution Stain
• Based on Kleihauer – Betke procedure.
• Acid pH will dissolve Hemoglobin A from red cell. Hemoglobin F is resistant to denaturation and remains in cell.
• Stain slide with eosin. Normal adult cells appear as “ghost” cells while cells with Hb F stain varying shades of pink.
• Useful way to differentiate between pancellular HPFH and heterocellular HPFH.
Routine Chemistry Tests
• Indirect bilirubin elevated in thalassemia major and intermedia
• Assessment of iron status, total iron binding capacity and ferritin level important in differentiating thalassemia from iron deficiency anemia.

No comments:
Post a Comment